With the development of life sciences and technology, peptide drugs are more and more valued by drug development companies due to their high efficacy, small dosage and small toxic and side effects. At present, there are more than 50 peptide drugs on the market, and about 140 peptide drugs are in clinical research, while the number of peptide drugs in the preclinical development stage has reached 500 to 600.
Like small molecule drugs, the pharmacokinetics of peptides are an important part of peptide drug development. However, unlike small molecule drugs, peptide drug bioanalysis has many difficulties, such as poor sensitivity and adsorption phenomena.
Waters® has been an industry leader in peptide bioanalysis, and has a complete solution for peptide bioanalysis, from highly efficient liquid phase separation (UPLC®) to ultra-high sensitivity and A wide scanning range (2-2048 amu) mass spectrometry (Xevo® TQ-S) and a sample preparation technology (µElution) with concentration without having to dry can solve various difficulties in the analysis of peptide substances.

Thymosin a1 is a therapeutic polypeptide (molecular formula C127H213N33O5, consisting of 29 amino acids), which is mainly used for the treatment of chronic hepatitis B and can also be used for vaccine immune response. Using Waters's total peptide analysis solution (Table 1), a bioanalytical method for thymosin a1 was established with a minimum detection limit of 0.5 ng / mL (S / N = 11) and a sample injection of 3 uL (Figure 1) Linear in the range of 0.5-250ng / mL, r2> 0.99 (weighting factor 1 / x2).

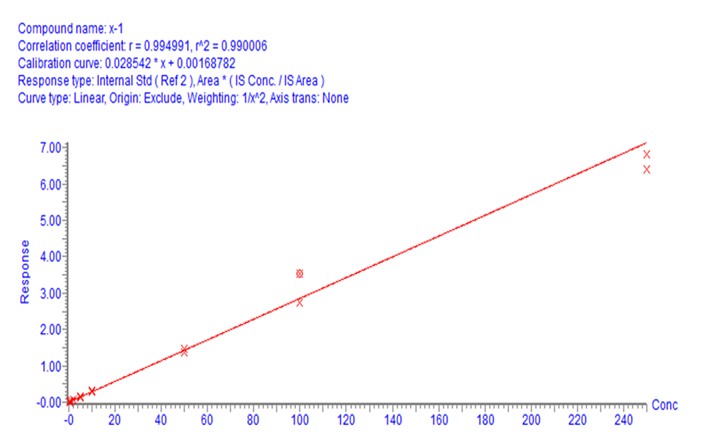 Figure 1 The signal-to-noise ratio and linear fitting curve of thymosin a1 at the lower limit of quantitation
Figure 1 The signal-to-noise ratio and linear fitting curve of thymosin a1 at the lower limit of quantitation
Ningbo XISXI E-commerce Co., Ltd , https://www.petspetstoys.com