Methanesulfonic acid is usually used as a raw material in the production of medicines and pesticides. It can also be used as a dehydrating agent, paint curing accelerator, fiber treatment agent, solvent, catalyst for carboxylation, esterification and polymerization. The presence of the impurity ions chloride ion and sulfate in the methanesulfonic acid reagent will affect the performance of the product and the safety of the equipment, and methanesulfonic acid will use sulfuric acid and chlorine-containing substances in the production process, and chloride ion is bound to exist And sulfate and other impurities remain, so the detection of chloride and sulfate in the high-purity raw material reagent methanesulfonic acid has become a necessary and necessary link in the production process. Traditional wet chemistry [1] can quantitatively detect constant chloride ions and sulfate radicals, but in trace detection, the detection error brought by wet chemistry is relatively large. Ion chromatography has been reported for the detection of chloride and sulfate in methanesulfonic acid [2], but some experimental details have not been elaborated in the literature.
1 Experimental part
1.1 Instruments and reagents
IC6100 ion chromatograph (Anhui Wanyi Technology Co., Ltd.); ultra-pure water instrument (Anhui Wanyi Technology Co., Ltd.);
Finnpipette pipette (20-200μL, 100-1000μL, 0.5-5mL) (ThermoFisher, USA), disposable 1mL syringe (Changzhou Jinlong Medical Plastic Equipment Co., Ltd.), organic phase needle filter (13mm × 0.22μm) (Tianjin Bona Ager Technology Co., Ltd.), 50mL volumetric flask, 100mL volumetric flask.
Na2CO3 (reference reagent, Tianjin Guangfu Technology Development Co., Ltd.) NaOH (superior grade, Tianjin Guangfu Technology Development Co., Ltd.), NaCl (reference reagent, Tianjin Guangfu Technology Development Co., Ltd.), anhydrous Na2SO4 (reference reagent, Tianjin) Guangfu Technology Development Co., Ltd.).
1.2 Chromatographic conditions
Wanyi WY-Anion-1 anion analysis column (4 × 250mm) and its WY-AG-1 protection column (4 × 50mm), conductivity detector, anion suppressor (Anhui Wanyi Technology Co., Ltd.), suppression current 4mA , Eluent: 3.6mmol / LNa2CO3 solution, flow rate 0.8mL / min, loop 100μL, oven temperature 45 ℃, detection cell temperature 50 ℃.
1.3 Preparation of standard solution
1.3.1 Preparation of standard stock solution
NaCl and anhydrous Na2SO4 reagent were baked at 105 ℃ to constant weight;
Accurately weigh 0.1649g of NaCl reagent, dissolve it with secondary deionized water, transfer to a 100mL volumetric flask, and dilute to the mark to obtain a 1000-mg / L Cl-standard stock solution;
Accurately weigh 0.1749g of Na2SO4 reagent, dissolve it with secondary deionized water, transfer to a 100mL volumetric flask, and bring the volume to the mark to obtain 1000mg / L of SO42-standard stock solution.
1.3.2 Preparation of linear solution
Pipette Cl- and SO42- standard stock solutions 1.0mL to 100mL volumetric flasks respectively, and dilute to the mark with secondary deionized water to obtain a mixed standard solution of 10mg / L;
Pipette 2.5, 1.25, 0.5, 0.25 and 0.05mL of 10mg / L mixed standard solution with a pipette, respectively, and put them into five 50mL volumetric flasks respectively, and dilute to the mark with secondary deionized water to get 0.5, 0.25 , 0.1, 0.05 and 0.01mg / L linear solution.
1.4 Sample preparation
Preparation of 15g / L NaOH solution: accurately weigh 1.5g of superior grade pure NaOH into a 100mL volumetric flask and make up to the mark with secondary deionized water;
Before using disposable syringe filters, use 3mL secondary deionized water for activation;
Pipette 0.1ml methanesulfonic acid sample accurately, transfer to 100mL volumetric flask, add 4ml15g / L NaOH solution, adjust PH value between 7-8, dilute to 100mL with secondary deionized water, 0.22μm disposable The syringe filter enters ion chromatography for analysis.
2 Results and discussion
2.1 Selection of PH value of sample solution
Add 2, 4, and 6 mL of 15 g / L NaOH solution to 0.1 mL of methanesulfonic acid sample respectively, and dilute to 100 mL with secondary deionized water, and then measure the pH values ​​of 4.8, 7.5, and 10.2, respectively, for three different types. The sample solution of PH value was analyzed by injection, and it was found that the retention time of chloride ion and sulfate in the sample solution with PH value of 4.8 was delayed by 2min compared with the retention time of chloride ion and sulfate in the standard solution, and the sample solution with PH value of 10.2 The retention time of chloride and sulfate in medium is 1.5min earlier than that of chloride and sulfate in standard solution. The retention time of chloride and sulfate in sample solution with pH value of 7.5 and chloride and sulfate in standard solution The retention time is very close, this is because the sample solution has too much acid or alkali to affect the elution of the eluent, so the difference in retention time will be too large. Therefore, choose to add 4ml15g / L NaOH solution, adjust the PH value between 7-8, can accurately determine the chloride ion and sulfate.
2.2 Selection of eluent concentration
Choose 2.4, 3.6 and 4.8 mmol / L sodium carbonate, respectively, as the eluent, and find the actual sample. Under the chromatographic conditions of 4.8 mmol / L and sodium carbonate, the resolution of methanesulfonic acid and chloride ion is less than 1.5 , Did not reach the baseline separation, affecting the accurate quantification of chloride ions in the actual sample; and using 2.4mmol / L sodium carbonate as the eluent, the resolution of methanesulfonic acid and chloride ions can reach 2.3, but the peak time of sulfate is 37min, which is not conducive to the improvement of analysis efficiency; under the chromatographic conditions of 3.6mmol / L sodium carbonate as the eluent, the resolution of methanesulfonic acid and chloride ion is 1.9, which achieves baseline separation, and the retention time of sulfate is Within 25min. Therefore, 3.6 mmol / L sodium carbonate solution was selected as the eluent, which not only prevented chloride ion and detection from being disturbed by high concentration of methanesulfonic acid, but also ensured that sulfate peaks appeared in a short time. Under this leaching condition, the standard mixed solution of chloride and sulfate was analyzed to obtain the following spectrum, as shown in Figure 1.
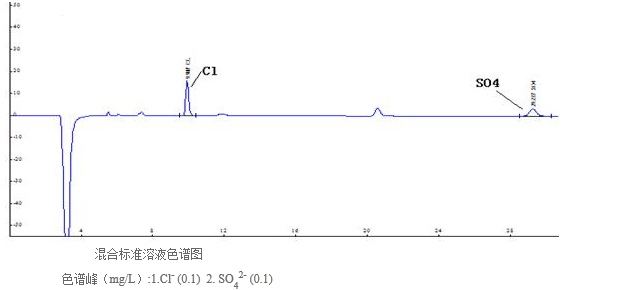
2.3 Linear range, detection limit and quantitation limit
Under the selected chromatographic conditions, the measured chloride ion and sulfate concentration between 0.01 ~ 0.5mg / L, the linear relationship is good. Samples of Cl- and SO42- mixed standard solutions with concentrations of 0.01, 0.05, 0.1, 0.25 and 0.5 mg / L were analyzed to obtain the peak areas of Cl- and SO42- at different concentrations. The concentration (mg / L) is plotted on the abscissa and the peak area is plotted on the ordinate. The linear equations for Cl- and SO42- are Y = 1.235 × 106X 2.432 × 104 and Y = 8.691 × 105X 4608, linear correlation coefficient r is 0.9999. The standard addition method was used to determine the detection limits of Cl- and SO42- in methanesulfonic acid samples with a signal-to-noise ratio (S / N) of 3, respectively, 0.12 and 0.45 μg / L, and a signal-to-noise ratio (S / N) of 10 The quantification limits of Cl- and SO42- in methanesulfonic acid samples were determined to be 0.396 and 1.485 μg / L, respectively.
2.4 Precision
Under the chromatographic conditions selected in 1.2, the standard mixed solution of 0.1mg / L was injected 6 times continuously, and the relative standard deviations of the peak areas of chloride and sulfate were 0.35% and 0.30%, respectively.
2.5 Actual sample detection and method recovery
After pre-treating the sample according to 1.4, the actual sample is tested under the chromatographic conditions selected in 1.2, and the detection spectrum of chloride and sulfate in high-purity methanesulfonic acid is obtained, as shown in Figure 2, according to the external standard method The chloride ion and sulfate in the actual sample were quantified to obtain the chloride ion and sulfate content in different methanesulfonic acid samples, see Table 1. In different samples, add 0.05mg / L of mixed standard solution respectively to perform recovery test by adding the standard method. The recovery results are shown in Table 1.
3 Conclusion
According to the characteristics of methanesulfonic acid and other high-purity reagents, this method designs a pretreatment method. The sample processing process is simple and easy to operate. At the same time, the entire analysis process is completed within 25 minutes, which can meet the process control requirements of enterprises in the production of methanesulfonic acid. Production and quality inspection play a supervisory role.
references:
[1] He Xiaowei, Chen Yongjie, etc. Determination of chloride ion in copper foil electrolyte by potentiometric titration. Metallurgical analysis, 2005, 25 (1): 87 ~ 88.
[2] Feng Chunxia, ​​Zang Qiuxia, etc. Determination of Cl- and SO42- in methanesulfonic acid by ion chromatography. Hebei Chemical Industry, 2005, 5:76.
Compostable Knife,Bioplastic Knife,Bioplastic Knife Cutlery,Compostable Cornstarched Knife
Anhui Jianfeng Environmental Protection Technology Co., Ltd , https://www.ahbiocutleries.com