Kamaishu (Shanghai) Biotechnology Co., Ltd. introduces you to the three common questions and answers of immunohistochemistry experiments
1. The background is too dark after staining, how to distinguish between specific and non-specific staining?
Full coloring means that the entire slice is stained with color, and the intensity of the coloring can be dark or light. In short, it is not clear which tissues are positive and those tissues are negative. The reasons for this phenomenon are:
(1) The antibody concentration is too high: the primary antibody concentration is too high is one of the common reasons. The solution is to test the working concentration of each new antibody before using it, so that each antibody can be personalized and find the ideal working concentration for its own laboratory, even if it is a ready-to-use antibody, it ca n’t be simple According to the instructions for dyeing.
(2) The antibody incubation time is too long or the temperature is high: The solution is to strictly implement the operating procedures. It is best to wear a timepiece or clock with you to remind you in time to avoid prolonging the time due to forgetting. The popular two-step method (Polymer) is highly sensitive, requiring the primary antibody incubation time to be not 1 hour, but 30 minutes. Therefore, it should be adjusted according to the staining result.
(3) DAB deterioration and color development time is too long: DAB is best used now, if there is sediment, it should be filtered before use. The prepared DAB should not be stored for too long, because in the absence of enzymes, hydrogen peroxide will also release oxygen atoms and react with DAB to reduce the effectiveness of DAB. Unused DAB is stored in the refrigerator for a few days. It is not advisable to use this seemingly economical method. The color development of DAB is best monitored under a microscope, and the reaction is terminated immediately when the desired degree of staining is reached. However, when there are too many stained sheets or when using a staining machine, this seems unrealistic, but at least some new or less used antibodies should be monitored for color development to avoid excessive color development time.
(4) Dried tissues: Failure to replenish the fluid in a timely manner after the repair fluid overflows, too many stained slices, too slow movements, forgetting to drip, and dripping of the drip are all reasons that cause the tissue to dry out. The solution is to operate carefully, using DAKO pen or PAP Pen to draw a circle around the tissue, which can effectively avoid the loss of liquid and increase the operation speed.
(5) The immersion time of the slice in the buffer or repair solution is too long (more than 24 hours): the reason is not clear, but the phenomenon exists. Some laboratories like to dewax the slices to repair the day before, and add antibodies for immunohistochemical staining the next day. If the container with slices and repair solution is placed in a 4oC refrigerator overnight, there is no obvious effect on the results. At room temperature, especially on hot summer days, background coloring will appear, so it should not be stored for too long.
(6) Polyclonal antibodies with degraded primary quality and poor quality: Pay attention to the expiration date of the antibody. The expired antibody is either not colored or the background is colored. When using a newly purchased antibody, it is best to set up a positive control and compare it with the used antibody.
2. After the primary antibody is taken out from 4 degrees, why do we need to re-warm it at 37 degrees?
(1) On the one hand, prevent the slices from being put into PBS easily from 4 degrees to take off the slice;
(2) On the other hand, make antigen-antibody binding more stable. Generally not needed, but may be useful for weakly expressed antigens. The molecular motion is different at 4 degrees and 37 degrees. The former has a lower probability of collision and faster movement than the latter. The latter combines faster, but the sensitivity is also improved and easy Causes non-specific staining.
(3) Actually, I agree with the latter statement, because I tried to take the liver or testicle slices from 4 degrees overnight and washed them directly with PBS.
3. What are the reasons for the release?
(1) The quality of polylysine slides. I originally bought it, and it is said that the new brand is also good, but what it looks like. It is better to supplement the film made by the pathology teacher used in the second batch later.
Filling And Linking Technology
Helper has designed different filler series for small scale producer, medium sized business, and industrial enterprises,such as vacuum filler,piston stuffers.
---The vane cell feed system, together with vacuum pumping system, guarantees a long service life and the highest product quality.
---Mechanical filler is newly designed by our feeding technical team and it represents a new beginning of Chinese mechanical filling machine.
---Pneumatic filler is the most economical solution for the beginning of small scale producer with frequent changes in the product range.
sausage linking system,sausage hanging system:
The sausage linking and hanging system is the advanced solution to hang the sausages immediately after twisting-off. The workers handle easily the hanged sausages to smoking step by using a hanging stick. A simple twist linking device is also available to the filling machine to realize automatic twisting products production.
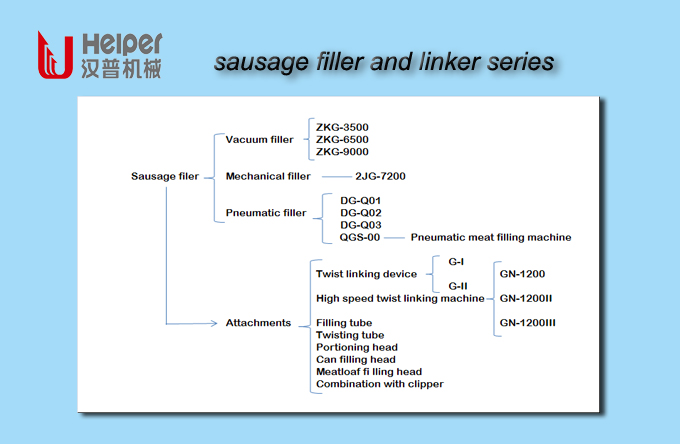
Automatic Sausage Stuffer,Vacuum Filler,Sausage Linking System,Sausage Hanging System,Piston Stuffers
Helper Machinery Group Co., Ltd. , https://www.helperpastamachine.com